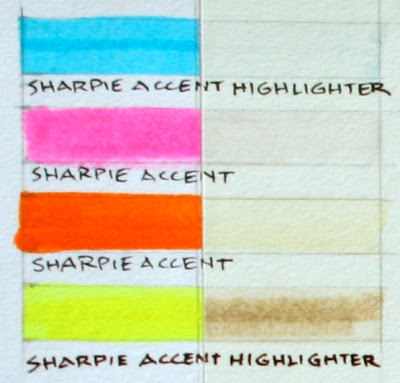
 Highlighter markers fare very poorly. Fluorescent highlighters use relatively unstable colorants that convert invisible ultraviolet light into light that you can see.
Highlighter markers fare very poorly. Fluorescent highlighters use relatively unstable colorants that convert invisible ultraviolet light into light that you can see. That conversion of UV to visible light adds light; that’s why a yellow highlighter stripe can appear lighter than the white of the paper. But the effect lasts only as long as the molecules hang together.
The blue, pink, and orange highlighters vanished, and the yellow highlighter darkened to a brown.
 Regular art markers, like these Berol Prismacolor brand Markers, didn’t do well. They faded away to the palest tints.
Regular art markers, like these Berol Prismacolor brand Markers, didn’t do well. They faded away to the palest tints. For this reason, if you have a marker drawing that you like, don't leave it exposed to light for very long. Put it in a drawer or between the pages of a book!
Why Do Colors Fade?
The reason colors fade is that the colorant molecules break down when they are exposed to light, especially to the shorter wavelengths of ultraviolet light, which pack more energy.
Ultraviolet light breaks the chain-like color particles into pieces, like a hammer smashing a necklace. The molecular fragments bond with oxygen to form new molecules that no longer have the same color-absorption properties.
Don’t worry: the news gets better from here on in.
